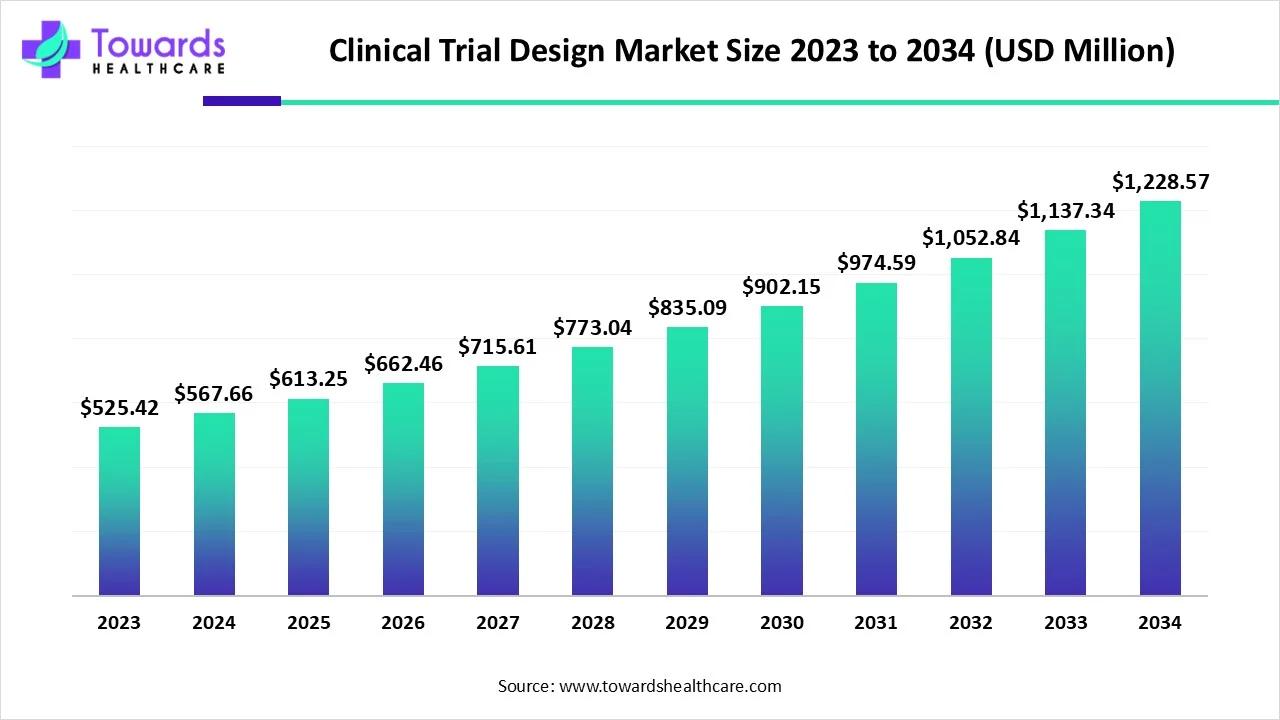
Clinical Trial Design Market Size to Gain USD 1,228.57 Million by 2034
The global clinical trial design market size is calculated at USD 613.25 million in 2025 and is expected to reach around USD 1,228.57 million by 2034, growing at a CAGR of 8.04% for the forecasted period.
Ottawa, Oct. 13, 2025 (GLOBE NEWSWIRE) -- The global clinical trial design market size was valued at USD 567.66 million in 2024 and is predicted to hit around USD 1,228.57 million by 2034, rising at a 8.04% CAGR, a study published by Towards Healthcare a sister firm of Precedence Research.
The clinical trial design market is rising due to increasing demand for personalized medicine, growing prevalence of chronic and rare diseases, and rapid adoption of AI-driven, decentralized, and patient-centric trial models that enhance efficiency and accelerate drug development.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report @ https://www.towardshealthcare.com/download-sample/5528
Key Takeaways
- North America led the clinical trial design market in 2024.
- Asia-Pacific is estimated to grow at the fastest rate in the market during the forecast period.
- By phase on trial, the phase III segment held a dominant presence in the market in 2024.
- By phase on trial, the phase II segment is anticipated to grow at the fastest rate in the coming years.
- By type of services, the eCRF (electronic Case Report Form) segment registered its dominance over the global market in 2024.
- By type of services, the SAP (Statistical Analysis Plan) segment is expected to grow at the fastest rate between 2025 and 2034.
- By the therapeutics area, the oncological disorders segment held the largest share of the clinical trial design market in 2024.
- By therapeutics area, the neurological disorders segment is estimated to grow at the fastest rate in the market during the forecast period.
Market Overview:
The design of clinical trials is experiencing rapid growth as the health industry moves towards better patient-centred and directed research. The growing demand for personalized medicine, the continuing growth of chronic diseases, and a deluge of new drug development are pushing the adoption of advanced designs, including adaptive and decentralized trials, which allow for more exact data, lower development costs, and quicker confirmations of regulatory compliance, with pharmaceutical and biotechnology companies buying into the advanced trial markets with innovation.
- In addition, the most significant and most critical growth area in clinical trial design and development is modernization surrounding rare diseases and precision therapies that propel the need for adaptive designs and better optimization in clinical trials.
Existing clinical trials have been increasingly evolving towards hyper portals into real clinical evidence, real world trial evidence, integration of all the digital health, risk-based monitoring and barometer’s of whatever evidence knowledge collation possible. Thus, Product development partnerships between CROs and sponsors/funders, and regulatory groups are being created at high speeds in the quest to continuously create more agile and less costly models of clinical trial design.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Major Growth Drivers:
- Growing Demand for Personalized Medicine – As the focus on targeted therapies and precision medicine increases, adaptive and customized clinical trial designs are becoming more popular, which allow for faster and more accurate results.
- Rising Incidence of Chronic and Rare Diseases – As the global population rises and prevalence of cancer, cardiovascular diseases, and rare genetic diseases rise, there is pressure to use novel trial models to bring treatments to market faster.
- Technological Development in Clinical Research – In addition to utilizing AI/machine learning/big data analytics in clinical research as an asset for patient recruitment, trial monitoring, and real-world data analysis, will help fuel market growth potential.
- Growth in Decentralized, Designated or Virtual Clinical Trials – Enabled by remote patient monitoring, wearable medical devices, and telemedicine solutions, patient-centric trial designs and methodologies have emerged to reduce dropout, and increase engagement and involvement.
- Regulatory and Governmental Support – Supportive policies, research grants, and streamlined processes for regulatory approvals to use advanced trial-based methodologies, motivate pharmaceutical and biotechnology companies to invest.
- Collaboration between CROs and Sponsors – Allowing and enabling sponsors and CROs together to access expertise, infrastructure, and the ability to run effective and quick Clinical Trials on a global scale.
Become a valued research partner with us - https://www.towardshealthcare.com/schedule-meeting
Key Drifts:
What Are the Major Trends Affecting the Clinical Trial Design Market?
The clinical trial design market is changing rapidly driven by trends that are transforming the methods and modalities of research. One of the most visible trends is the move toward adaptive trial designs allowing more flexibility in making adjustments to the protocol to improve efficiency and results. The other trend we see is strong consumer shift inward toward decentralized and virtual, driven again by telemedicine and consumer wearable devices coupled with remote monitoring that allow patient's increased accessibility and retention.
Further, the increasing application of artificial intelligence (AI) and big data analysis techniques is changing the face of trial optimization and improving patient recruitment, predicting outcomes and accelerating data-driven decisions. Finally, real-world evidence (RWE) and patient centricity is also impacting the designs by providing ways to better understand the most relevant, cost-effective and timely ways to obtain results. All of these trends suggest an improving future will mean clinical trials designs will be substantially smarter and more agile and innovation-focused.
Significant Challenge:
What are the Key Challenges in the Clinical Trial Design Market?
The clinical trial design market has seen rapid growth over the last few years, however there will always be challenges to continuous growth. The costs and complexities of conducting innovative trials can be seen as the most significant barrier as they often require significant infrastructure and skills just to leave the starting line. Patient recruitment and retention continue to be an issue, particularly in the rare diseases market where the pool of eligible patients will always be limited.
Regulatory hurdles and challenges such as the different compliance standards across different countries can result in additional delays to execution and an increase in complexity. Concerns around data privacy, especially within decentralized and digital trials, can compound operational hurdles. Trial timelines and the risk of trial failures impose a significant financial burden on Sponsor Organizations and Contract Research Organizations. Overcoming these prevention barriers will require more rational processes and stronger collaborative frameworks.
Regional Analysis:
Why is North America the leader in the Clinical Trial Design market?
The clinical trial design market is the largest in North America, owing to North America's established healthcare infrastructure, presence of global pharma players, and an adaptable regulatory system. North America also has high R&D spending, fast adoption of digital health technologies, growing talent pool and deep roots in innovation, all of which provide a strong influence in the design of clinical research.
Download the single region market report @ https://www.towardshealthcare.com/checkout/5528
What Makes Asia Pacific Rapidly Grow in Clinical Trial Design Market?
Asia-Pacific is the fastest growing clinical trial design market due to the region's significant pool of patients, the cost to execute a trial, and increasing government support for research. Also, clinical collaborations are increasing and the time it takes to recruit patients is decreasing, and decentralized trials are becoming more widely adopted by the research community, creating an effective and rapidly growing market for Global sponsors.
Segmental Insights:
By phase on trial:
Why Phase III dominates in the Clinical Trial Design Market?
Phase III dominates the clinical trial design market because of its broad testing of many patients to verify safety, efficacy, and longitudinal outcomes. This phase is the central component for acquiring regulatory approvals and is equally expensive and strategically important for pharmaceutical and biotechnology companies worldwide.
Why Phase II is the fastest growing in the Clinical Trial Design Market?
Phase II is the fastest growing segment in the clinical trial design market as it is foundational to proving effectiveness and optimal dosing. Increased investment into innovative therapies, including drugs targeting cancers and rare diseases is driving demand for well thought out Phase II designs. The need for early patient perspectives accelerates this subsegment growth on a global basis.
By type of service:
Why is the eCRF Segment Dominant in the Clinical Trial Design Market?
The electronic case report form (eCRF) segment dominates the clinical trial design market because electronic CRFs cut down on data blanket and they improve accuracy and promote regulatory compliance. In today's digital environment with advancing technology, eCRFs can enable the development of faster data entry, real-time data monitoring, and improve patient safety, which provides the backbone to clinical study research design.
Why is SAP the Fasted Growing Segment of the Clinical Trial Design Market?
SAP, or statistical analysis plan, is growing fastest in the clinical trial design space because of increasing reliance by the pharmaceutical and biotech industry on statistical modelling to promote the reliability of clinical trials. As the clinical trials grow in complexity due to increased regulations where sponsors have regulatory obligations, SAP provides a transparent approach to improving the value of trial data for purposes of regulatory approval by improving interpretability, building trust facilitating a deep comprehension for evidence-based decision making across the global domains of clinical trial research.
Get the latest insights on life science industry segmentation with our Annual Membership: https://www.towardshealthcare.com/get-an-annual-membership
By therapeutic area:
What Makes the Oncological Disorders Segment the Largest in the Clinical Trial Design Market?
The oncology disorder segment holds the largest share of the clinical trial design market, due to an increasing worldwide cancer burden and need for new therapies. The large-scale impact of oncology trials is bolstered by significant R&D investments, precision medicine, and targeted therapies.
What Makes the Neurological Disorders Segment the Fastest Growing Segment in the Clinical Trial Design Market?
The neurological disorders segment is the fastest growing, fueled by increasing incidence of Alzheimer's Disease, Parkinson’s and rare neurological conditions. Neurology is experiencing great acceleration in research due to innovations in neuroimaging, biomarkers, and high versus low precision trials. Increased patient demand and unmet therapeutic needs are also leading to faster adoption of innovative trial design in neurology.
Browse More Insights of Towards Healthcare:
The North America central lab market is valued at USD 1,384 million in 2024 and is expected to grow to USD 1,463 million in 2025. Forecasts indicate the market will reach approximately USD 2,411 million by 2034, expanding at a CAGR of 5.71% between 2025 and 2034.
The Middle East and Africa clinical trials market recorded a size of USD 3.81 billion in 2024, set to increase to USD 4.07 billion in 2025, and is projected to reach nearly USD 7.36 billion by 2034, reflecting a CAGR of 6.80% over the forecast period.
The North America clinical trials market is estimated at USD 21.76 billion in 2024, growing to USD 22.52 billion in 2025, and is forecast to reach around USD 30.69 billion by 2034, with a CAGR of 3.50% between 2025 and 2034.
Europe’s central lab market was valued at USD 865 million in 2024 and is expected to rise to USD 914 million in 2025. By 2034, the market is projected to reach USD 1,507 million, growing at a CAGR of 5.71%.
The U.S. clinical trial imaging market reached USD 684 million in 2024 and is anticipated to grow steadily to USD 748.09 million in 2025, reaching USD 1,675.4 million by 2034, at a CAGR of 9.37%.
Globally, the clinical trial imaging market was valued at USD 2.41 billion in 2024 and is expected to grow to USD 2.62 billion in 2025, eventually reaching USD 5.58 billion by 2034, driven by a CAGR of 8.8%.
The global clinical trial central lab services market is projected at USD 4.13 billion in 2024, increasing to USD 4.54 billion in 2025, and expected to reach USD 10.54 billion by 2034, expanding at a CAGR of 9.84% between 2025 and 2034.
The global oncology clinical trial market stood at USD 13.64 billion in 2024, is forecast to grow to USD 14.36 billion in 2025, and is projected to reach nearly USD 22.85 billion by 2034, at a CAGR of 5.30%.
The global clinical trial software market was valued at USD 0.9 billion in 2024 and is expected to increase to USD 1.03 billion in 2025, reaching USD 3.23 billion by 2034, supported by a robust CAGR of 13.74% between 2025 and 2034.
The global HIV clinical trials market is estimated at USD 1.27 billion in 2024, growing to USD 1.36 billion in 2025, and projected to reach USD 2.54 billion by 2034, expanding at a CAGR of 7.17% between 2025 and 2034.
Recent Developments:
- On September 3, 2025, the U.S. FDA proposed a new pathway to expedite the approval of drugs targeting rare diseases affecting fewer than 1,000 people in the U.S. This process may accept data from single-arm clinical trials when supported by confirmatory evidence, such as mechanistic rationale or pre-clinical findings
- On August 21, 2025, Japanese pharma company Takeda is evaluating the possibility of conducting global trials in India to speed drug launches. This initiative seeks to capitalize on India’s broad patient base, cost advantages, and growing hospital infrastructure. Takeda also launched an innovation center in Bengaluru, aiming to scale its AI-enabled R&D and increase digital workforce from ~500 to 750.
Top Players of Clinical Trial Design Market:

- Parexel
- Charles River Laboratories
- IQVIA
- Medpace
- Eli Lilly
- Syneo Health
- WuXi AppTec
- Pfizer
- Labcorp
- Novo Clinical
- Merk & Co
Download the Competitive Landscape market report @ https://www.towardshealthcare.com/checkout/5528
Segments Covered in the Report
By Phase of Trial
- Phase I
- Phase II
- Phase III
- Phase IV
By Type of Services
- eCRF (electronic Case Report Form)
- SAP (Statistical Analysis Plan)
- Site of identification and selection
- Medical writing
- Other
By Therapeutic Area
- Oncological disorders
- Cardiovascular disorders
- Inflammatory disorders
- Neurological disorders
- Other therapeutics area
By Region
- North America
- U.S.
- Canada
- Asia Pacific
- China
- Japan
- India
- South Korea
- Thailand
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Sweden
- Denmark
- Norway
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa (MEA)
- South Africa
- UAE
- Saudi Arabia
- Kuwait
Immediate Delivery Available | Buy This Premium Research @ https://www.towardshealthcare.com/checkout/5528
Access our exclusive, data-rich dashboard dedicated to the healthcare market - built specifically for decision-makers, strategists, and industry leaders. The dashboard features comprehensive statistical data, segment-wise market breakdowns, regional performance shares, detailed company profiles, annual updates, and much more. From market sizing to competitive intelligence, this powerful tool is one-stop solution to your gateway.
Access the Dashboard: https://www.towardshealthcare.com/access-dashboard
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics, with a strong emphasis on life science research. Dedicated to advancing innovation in the life sciences sector, we build strategic partnerships that generate actionable insights and transformative breakthroughs. As a global strategy consulting firm, we empower life science leaders to gain a competitive edge, drive research excellence, and accelerate sustainable growth.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Europe Region: +44 778 256 0738
North America Region: +1 8044 4193 44
APAC Region: +91 9356 9282 04
Web: https://www.towardshealthcare.com
Our Trusted Data Partners
Precedence Research | Statifacts | Towards Packaging | Towards Automotive | Towards Food and Beverages | Towards Chemical and Materials | Towards Consumer Goods | Towards Dental | Towards EV Solutions | Nova One Advisor | Healthcare Webwire | Packaging Webwire | Automotive Webwire
Find us on social platforms: LinkedIn | Twitter | Instagram | Medium | Pinterest

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.

